After Scientific Breakthrough: Digid Seeks FDA Approval for Its Rapid Corona Antigen Test
- Written by ACN Newswire - Press Releases

|
|
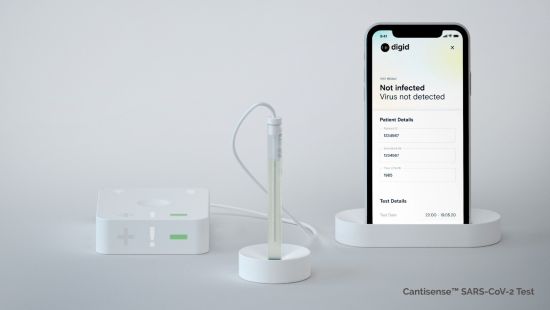
| Figure 1: Point-of-care test kit: sensor hub, biosensor, mobile device. With digid's point-of-care solution, the measurement results are transmitted wirelessly from a sensor hub to a mobile device. By connecting to a secure analytics platform, the numerous sensor data can be augmented with further data and processed anonymously for research and diagnostic applications. (prototype/copyright: Digital Diagnostics AG) |
In contrast to PCR testing, the new test provides a clear electronic "YES" or "NO" information within a few minutes, saving precious time in the diagnosis. What's more, the Digid Cantisense(TM) SARS-CoV-2 Test directly detects the presence of the virus while other rapid tests only recognize antibodies.
Konstantin Kloppstech, CTO at Digital Diagnostics, says: "Recent test series at the HZI high-security laboratory have shown that SARS-CoV-2 viruses can be detected directly using our Cantisense technology and without the need for PCR or further sample processing. This is a scientific breakthrough. We have coated cantilevers with a capture layer of highly specific monoclonal antibodies, which can reliably bind SARS-CO-2 viruses in the test fluid."
Constantin von Gersdorff, CEO of Digital Diagnostics, said: "The next step will be to initiate clinical studies with patient samples. To this end, we have already established international collaborations with leading hospitals."
With Digid's test kit, the measurement results are transmitted wirelessly from a sensor hub to a mobile device. By connecting to a secure analytics platform, the sensor data can be augmented with further data and processed anonymously for research and diagnostic applications.
Due to its measurement speed and the highly reliable results, the test is particularly suitable to support the containment of the current SARS-CoV-2 pandemic. The test offers the possibility of simple and rapid testing of patients and medical staff as an alternative to laboratory tests and enables reliable identification of infected persons within minutes. The possible areas of application therefore also include screening for access control at airports and railway stations, hospitals and specially protected areas (such as retirement homes) as well as for companies who want to ensure that their production runs smoothly and safely.
Digital Diagnostics has applied for approval of the Digid Cantisense(TM) SARS-CoV-2 Test by the US Food and Drug Administration (FDA). Digid aims to provide millions of units by July 2020. A further scaling of capacities is planned in the short term. The experts at Digital Diagnostics are also working at full speed to further develop the technology and to adapt the point-of-care testing procedure for use by consumers at home.
Press contactThomas Hubersemanticom GmbH+49 30 275 80 81 11digid-pr@semanticom.eu
Related Imageshttps://www.newsfilecorp.com/redirect/waAZCxPRPoint-of-care test kit: sensor hub, biosensor, mobile deviceWith digid's point-of-care solution, the measurement results are transmitted wirelessly from a sensor hub to a mobile device. By connecting to a secure analytics platform, the numerous sensor data can be augmented with further data and processed anonymously for research and diagnostic applications. (prototype/copyright: Digital Diagnostics AG)
Related Linkshttps://digid.com/en/
Copyright 2020 ACN Newswire. All rights reserved. www.acnnewswire.com
Authors: ACN Newswire - Press Releases
Read more //?#