Hua Medicine Successfully Completes SEED (HMM0301), Dorzagliatin's Phase III Monotherapy Trial
- Written by ACN Newswire - Press Releases
|
|
| Figure 1 |
|
|
| HbA1c Reduction *p |
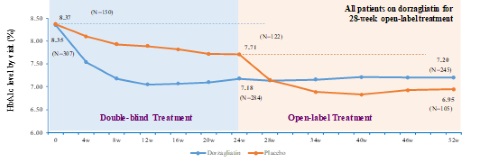
In November 2019, Hua Medicine announced the trial had achieved its primary efficacy and safety endpoints over the initial 24-week double blinded period. For the 52-week treatment period, the efficacy and safety profiles were sustained based on the topline data analysis. During the 28-week open-label period, patients initially receiving a placebo (i.e., the placebo group) were administered dorzagliatin for the first time. Figure 1 below illustrates the efficacy (as measured by HbA1c reduction) for the two-cohort groups for the entire 52-week period.
In the 28-week open-label treatment period, dorzagliatin continued to exhibit a safe and well-tolerated clinical profile. A safety analysis based on study safety population demonstrated that dorzagliatin was well tolerated and had a good safety profile. The incidence of adverse events was similar between the dorzagliatin-treated and placebo groups. There was less than 1% hypoglycemia with blood glucose
"We are incredibly proud of our accomplishment. The Hua Medicine team and our partners have worked closely together over the last decade to advance the development of dorzagliatin," said Dr. Li Chen, CEO and founder of Hua Medicine. "With the successful completion of SEED, we become the first company globally to advance a glucokinase activator through clinical development. This is an incredible achievement for the Hua Medicine team, Chinese investigators, Hua's partners and supporters, and most importantly, for Type 2 diabetes patients globally." On Sunday, June 14th, 2020, Dr Chen presented more comprehensive data of the 24-week double blinded, placebo-controlled period of SEED study at the ADA 2020 80th Scientific Sessions. In addition to reduced glucose levels, the data presented at the ADA indicated improved beta cell function for the dorzagliatin-treatment group (as measured by the clinically meaningful biomarker HOMA2-beta). In contrast, the placebo group experienced reduced beta cell function during the same period. Dr. Chen added: "Hua Medicine will continue its efforts to develop a potential disease modifying therapy to treat diabetes."
SEED (Efficacy and Safety Evaluation of Dorzagliatin) study designSEED is a randomized, double-blind, placebo-controlled Phase III study in 463 drug naive type 2 diabetes patients. Patients are treated with twice-daily doses of dorzagliatin (75 mg) or placebo, randomized 2:1. The clinical study evaluated the efficacy and safety of dorzagliatin during 24 weeks of double-blinded treatment, followed by a subsequent 28-week open-label treatment period, for a total of 52 weeks. During the 28-week open-label period, both patient groups were treated with twice-daily doses of dorzagliatin (75 mg). The trial was conducted in compliance with the guidelines of the Chinese Society of Endocrinology, which require physicians to educate patients and strictly enforce improved exercise and dietary control, as well as continuous self-monitoring, in treating Type 2 diabetes. The trial was conducted at 40 clinical sites across China led by Professor Dalong Zhu, President of the Chinese Diabetes Society.
About DorzagliatinDorzagliatin is an investigational first-in-class, dual-acting glucokinase activator, designed to control the progressive degenerative nature of diabetes by restoring glucose homeostasis in patients with Type 2 Diabetes. By addressing the defect of the glucose sensor function of glucokinase, dorzagliatin has the potential to restore the impaired glucose homeostasis state of patients with Type 2 Diabetes and serve as a first-line standard-of-care therapy for the treatment of the disease, or as a cornerstone therapy when taken in combination with currently approved anti-diabetes drugs.
About Hua MedicineHua is a leading, clinical-stage innovative drug development company in China focused on developing novel therapies for the treatment of diabetes. Founded by an experienced group of entrepreneurs and international investment firms, Hua advanced a first-in-class oral drug for the treatment of Type 2 Diabetes into NDA-enabling stage and is currently evaluating the therapy in adults with diabetes in two Phase III trials in China and various earlier stage clinical trials in China and the United States. Dorzagliatin has achieved its first primary endpoint in a Phase III monotherapy trial. The Company has initiated product life-cycle management studies of this novel diabetes therapy and advanced its use in personalized diabetes care. Hua Medicine is working closely with disease experts and regulatory agencies in China and across the world to advance diabetes care solutions for patients worldwide.
For more informationHua MedicineWebsite: www.huamedicine.comInvestorsEmail: ir@huamedicine.comMediaEmail: pr@huamedicine.com
Copyright 2020 ACN Newswire. All rights reserved. www.acnnewswire.com
Authors: ACN Newswire - Press Releases
Read more //?#